Abstract

Background Rusfertide (PTG-300) is a potent mimetic of hepcidin that binds to ferroportin causing it to be internalized and degraded, thereby decreasing iron availability in bone marrow and reducing aberrant erythrocytosis. In the phase 2 REVIVE study (PTG-300-004; NCT04057040) of patients with polycythemia vera (PV), rusfertide treatment resulted in sustained control of hematocrit (HCT) at <45% and eliminated the requirement for therapeutic phlebotomy (TP) in 84% of patients. Rusfertide was well tolerated, with limited grade 3 treatment-emergent adverse events (TEAEs) and no grade 4 or 5 TEAEs [Hoffman, ASH 2021]. We present an analysis of the rusfertide TEAE profile in clinically relevant subgroups.
Methods Patients with TP-dependent (i.e., ≥3 phlebotomies within a 6-month period) PV were eligible for REVIVE, which was comprised of 3 parts: a 28-week open-label, dose-finding stage; a 12-week double-blind randomized withdrawal stage; and a long-term extension. In the dose-finding stage, subcutaneous rusfertide doses (10-120 mg) were administered with TP or with TP plus prior cytoreductive agents (CYR-T), with individualized dose titration to maintain HCT at <45%. This analysis focuses on the 70 patients enrolled in the study with ≥8 weeks of exposure to study treatment.
Results Forty-one patients (59%) were considered to be high-risk for thrombotic events because of age ≥60 years (n=26, 37%) or because of a prior thrombotic event (n=15, 21%). The TEAE profile was similiar in high-risk and low-risk patients. The TEAEs with the greatest difference in incidence between high-risk and low-risk patients, respectively, were fatigue (34% vs 21%), hyperuricemia (5% vs 17%), rash (12% vs 0), and paresthesia (15% vs 3%). The incidence of grade 3 TEAEs (11% vs 15%) was similiar in patients receiving rusfertide with TP (n=36) compared to those receiving rusfertide with TP plus CYR-T (n=34). The incidence of all-grade TEAEs was also similar except for a greater incidence of contusions (14% vs 0), and fatigue (33% vs 24%) in the rusfertide with TP group, and a greater incidence of dyspnea in the rusfertide with TP plus CYR-T group (18% vs 8%). There were no notable differences in injection site reactions in any of the subgroups or in any TEAEs by rusfertide dose.
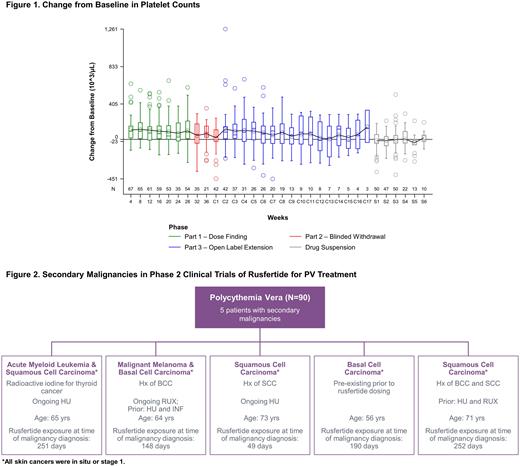
Rusfertide controlled HCT and decreased red blood cell counts. This was often accompanied by small (<20%) and clinically insignificant changes in platelet (PLT) counts that returned to baseline when rusfertide was stopped (Figure 1). However, 6 (9%) patients (3 male, 3 female) reported a PLT count >106/µL and a >20% increase from baseline on ≥1 consecutive assessment with most (n=5) being in the rusfertide with TP group. Of these 6 patients, 4 were categorized as high-risk and 2 as low-risk. In most cases, the PLT elevations occurred by week 4 and stabilized over the course of treatment. The PLT elevations were not associated with increasing rusfertide dose or duration, or with clinical sequelae such as bleeding, thrombosis, or disease progression. Across all rusfertide phase 2 PV trials (N= 90), 18 patients had a history of pre-existing cancers at baseline, of which 9 had a history of non-melanomatous skin cancers (NMSC). After beginning treatment with rusfertide, secondary malignancies were identified in 5 patients (5.5% of patients) (Figure 2). All 5 patients had prior concomitant cytoreductive therapy with HU or Rux, each of which is associated with an increased risk of NMSC [Verner, Leuk Lymph 2014; Lin, J Am Acad Dermatol 2022]. One had a pre-existing lesion confirmed as an in situ NMSC upon protocol mandated biopsy.
Conclusions The TEAE profile of rusfertide is consistent across subgroups defined by thrombotic risk and concomitant CYR-T, with no clear differences in TEAE profile. While a small proportion of patients developed thrombocytosis, it was asymptomatic, stabilized over the course of treatment, and did not increase with higher rusfertide dose and treatment duration. Reactive thrombocytosis may have resulted from localized iron deficiency [Kasper, JAMA 1965]. Importantly, it was not associated with clinical sequelae. Consistent with previous reports [Pemmaraju, Blood 2019], secondary malignancies (6 early-stage skin cancers and 1 AML) were observed in 5 patients, all with prior history of malignancy or cytoreductive therapy. These data suggest that the rusfertide TEAE profile is manageable across the range of PV patients.
Disclosures
Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Verstovsek:Roche: Research Funding; Genentech: Research Funding; Sierra Oncology: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; CTI BioPharma Corp.: Research Funding; Promedior: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Protagonist Therapeutics: Research Funding; PharmaEssentia: Research Funding; Novartis: Consultancy, Research Funding; ItalPharma: Research Funding; Constellation Pharmaceuticals: Consultancy; Celgene: Consultancy, Research Funding; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Kremyanskaya:Incyte: Consultancy, Research Funding; Ionis: Research Funding; Kura: Research Funding; Kronos: Research Funding; Chimerix: Research Funding; BMS: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Protagonist Therapeutics: Consultancy, Research Funding. Valone:Protagonist Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Modi:Protagonist Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Khanna:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. O'Connor:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gupta:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hoffman:Protagonist Therapeutics: Consultancy; Turning Point: Research Funding; Novartis: Research Funding; Silence Therapeutics: Consultancy; Novartis: Other: Chair DSMB; Scholar Rock: Research Funding; Repare: Research Funding; Ionis: Consultancy; Abbvie: Other: Chair DSMB, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal